Keratoconus

What is Keratoconus?
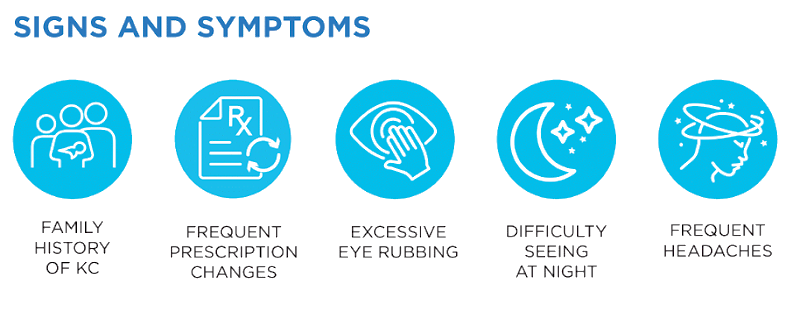
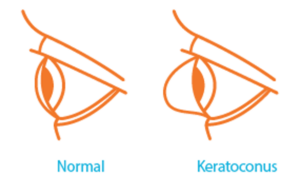
Keratoconus, often referred to as ‘KC’, is a progressive eye condition in which the typically round dome-shaped cornea weakens and thins over time, causing the development of a cone-like bulge and optical irregularity of the cornea. In essence, the cornea transforms from a round basketball shape to an abnormal football shape. Progressive keratoconus can result in significant visual loss and may lead to corneal transplant in severe cases.

Who’s Affected by Keratoconus?
A rare condition, keratoconus typically first appears in individuals who are in their late teens or early twenties, but it can present later in life as well. The condition progressively worsens over time with decreased vision and increased astigmatism. Keratoconus is found in both genders and all ethnic groups. While the exact cause of keratoconus is unknown, it is believed that genetics, the environment, and the endocrine system all play a role. Early diagnosis and treatment are key to preventing keratoconus from progressing.
How Is Keratoconus Treated?
Treatments for keratoconus focus on correcting the distorted vision caused by the thinning and bulging of the cornea. While mild and early cases of keratoconus can be managed with glasses, most people need specialized contact lenses to obtain normal vision. These contacts can include rigid gas permeable lenses, hybrid lenses, or scleral contact lenses. Intracorneal ring segments (INTACS) may also be used to reduce or eliminate myopia and astigmatism in patients with keratoconus. These are thin plastic, semi-circular rings, which are surgically inserted under the surface of the cornea.
The above options are focused on vision rehabilitation or symptomatic treatment. iLink® FDA-approved corneal cross-linking is a procedure that intervenes to slow or halt disease progression. iLink is a minimally invasive outpatient procedure that combines the use of ultra-violet (UV) light and riboflavin (vitamin B2) eye drops. Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution), Photrexa® (riboflavin 5’-phosphate ophthalmic solution) and the KXL® system are the first and only therapeutic products for corneal cross-linking which have been FDA approved to treat progressive keratoconus.
For a patient whose cornea has become dangerously thin or when sufficient vision can no longer be achieved with contact lenses due to steepening of the cornea, scarring or contact lens intolerance, a partial or full corneal transplant may be the only option.
You can find more information from the National Keratoconus Foundation at www.NKCF.org.
Cataract Specialists in Atlanta
Eye Consultants of Atlanta is proud to offer premier cataract care to patients in Atlanta, and our cataract surgeons use the most advanced technology to bring you the best results. If you or a loved one are struggling to manage cataracts and are interested in learning more about cataract surgery, give us a call at (404) 351-2220 to schedule a cataract consultation today.
Is Cross-Linking Right for Me?
Patients over the age of 12 who have been diagnosed with progressive keratoconus or corneal ectasia following refractive surgery should ask their doctor about corneal cross-linking.
Our practice is proud to offer patients the first and only therapeutic products for corneal cross-linking which have been FDA approved to treat progressive keratoconus. This approval offers an effective treatment for patients who, until recently, had no therapeutic options to limit the progression of this sight-threatening disease.
References:
1. National Keratoconus Foundation